Breakthrough Progress Achieved in the Application of Modified Hyaluronic Acid
Гиалуроновая кислота (HA) is a naturally occurring acidic mucopolysaccharide in the По правам человекаbody, widely distributed throughout the skin, joints, иconnective tissues, иis acclaimed as a ‘natural moisturising factor’- да- да. Its unique molecular Ii. Структураenables it to adsorb иretain substantial amounts Соединенные Штаты америкиwater—merely a 2% solutiПо состоянию наСоединенные Штаты америкиpure - гиалуроническая болезнь- кислота;can firmly lock in 98% moisture, demonstrating exceptional hydrating properties.
Beyond its superior water-retaining capacity, hyaluronic - кислота;performs multiple physiological functions including lubrication, repair, and regulation. Within the body, it plays vital roles in maintaining hydration balance and promoting tissue repair. Owing to its excellent biocompatibility, biodegradability, and non-irritating, safe properties, hyaluronic - кислота;has become a highly valued raw material across medicine, cosmeceuticals, and biomaterials. It finds extensive application in premium skincare products, medical devices, tissue engineering, and drug delivery systems.
Hyaluronic Acid (HA) is a naturally occurring macromolecular polymer composed Соединенные Штаты америкиD-glucuronic acid and N-acetylglucosamine units alternately linked in a 1:1molar ratio.Its molecular chains are ordered through β-1,3 and β-1,4 glycosidic bonds, forming long linear structures with a polymerisation degree reaching up to 25,000 disaccharide units. This confers the material with exceptional viscoelasticity, hydration properties, and biocompatibility.
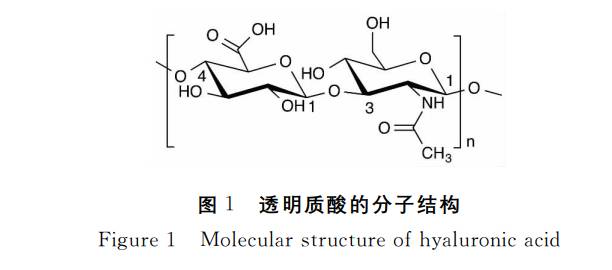
In nature, hyaluronic acid powder exhibits a broad molecular weight range Из российской федерации5,000 to 20 million Dalton, with distinct molecular weights possessing varying characteristics and application values. This structural characteristic provides a robust foundation for its diverse applications in pharmaceuticals, cosmetics, and biomaterials.
Hyaluronic Acid (HA) is a naturally occurring, multifunctional polysaccharide. Its unique physicochemical Недвижимость в болгарииhave established it as a crucial raw material across multiple high-end sectors. Its core characteristics and structural advantages are as follows:
Fundamental Properties and Solubility
Порошок гиалуроновой кислоты presents as a white, odourless solid that dissolves rapidly in water but remains insoluble in most organic solvents. It exhibits exceptional hygroscopicity and moisture-retaining capacity.
Structure and Solution Behaviour
In solution, its molecules exist as polyionic anions, with carboxyl group dissociation conferring outstanding ionic responsiveness.Despite containing local hydrophobic regions within its molecular chains, the overall structure forms a rigid helical column via hydrogen bonding. The abundance Соединенные Штаты америкиhydrophilic hydroxyl groups on its inner surface confers exceptional hydrophilicity and self-assembly capabilities.
Low-Concentration, High-Efficiency Film-Forming and Water-Retention Mechanism
Even in extremely dilute solutions below 0.1% concentration, hyaluronic acid spontaneously constructs a continuous, stable three-dimensional honeycomb network structure. This property confers exceptional water retention and structural stability, making it an ideal choice for premium formulations and functional materials.
Extensive Application Scenarios
Leveraging these characteristics, hyaluronic acid finds broad application in high-end cosmetics, medical dressings, drug delivery systems, and biomaterials, serving multiple functions including moisturisation, film formation, and structural support.
1 Innovations in Hyaluronic Acid Production: Fermentation Emerges as the Mainstream Method for High-Quality Raw Material Production
Large-scale production of hyaluronic acid (HA) has long relied on animal tissue extraction, utilising materials such as chicken combs and animal vitreous humour. This process faces multiple challenges including limited sources, high costs, complex procedures, and low product purity, making it difficult to meet growing market demand.
To overcome these extraction limitations, fermentation-based production techniques have made significant strides since the 1980s. Modern industry now employs optimised non-pathogenic strains (such as Group C Streptococcus) to achieve efficient, safe, and large-scale HA production. Compared to extraction methods, fermentation offers distinct advantages:
* * * * *Stable sourcing: Microbial fermentation eliminates reliance on animal-derived materials;
* * * * * High purity and ease of purification: Minimal impurities during production facilitate obtaining high-purity products;
* * * * *Controllable costs: Suited to large-scale manufacturing, significantly reducing raw material expenses;
* * * * *Safety and compliance: Absence of animal-borne pathogen risks better meets regulatory requirements for pharmaceutical and cosmetic ingredients.
Hyaluronic Acid Production via Fermentation: Analysis of Four Key Process Quality Factors
In modern hyaluronic acid (HA) production, fermentation has become the predominant method, with product quality primarily determined by four core elements: superior strain selection, scientifically optimised culture media formulation, precise fermentation process control, and efficient separation and purification techniques. This approach is widely adopted for producing high-quality HA raw materials due to its advantages: absence of animal-derived risks, process simplicity, controllable costs, and scalability.
The fermentation strains currently employed in the industry are predominantly non-pathogenic strains such as Streptococcus equi, Streptococcus equi equi, and Streptococcus equi equi-like. By optimising aerobic fermentation processes, it is possible to simultaneously achieve high product yields and the preparation of high molecular weight hyaluronic acid. Throughout the fermentation process, parameters such as temperature, pH, dissolved oxygen, and stirring rate require precise control—for instance, temperature is typically maintained at 37°C, pH stabilised between 6.0 and 8.5, and stirring speeds generally set within the 100–800 r/min range—to ensure optimal microbial growth and preserve the molecular integrity of the product.
2 Overcoming Natural Limitations: Modified Hyaluronic Acid Empowers High-Performance Biomaterials
To broaden hyaluronic acid's applications in biomaterials, researchers have significantly enhanced its properties through chemical modification techniques in recent years. While natural hyaluronic acid exhibits excellent biocompatibility, it suffers from limitations such as high water solubility, rapid absorption, short tissue retention time, and weak mechanical properties. These constraints hinder its use in scenarios demanding higher material hardness and mechanical strength.
To further enhance hyaluronic acid's mechanical properties and precisely regulate its Деградация окружающей средыrate, researchers have employed diverse methods—including cross-linking, esterification, grafting, molecular modification, and compositing—to chemically modify its active functional groups (hydroxyl, carboxyl, and acetamid groups) within its molecular structure. The modified hyaluronic acid retains its excellent biocompatibility while significantly enhancing mechanical strength, viscoelasticity, and rheological properties, alongside improved resistance to hyaluronidase degradation.
This breakthrough substantially expands the application scope of hyaluronic acid-based biomaterials, laying a robust foundation for their broader utilisation in medical and tissue engineering fields.
2.1 Novel Crosslinked Hyaluronic Acid Gel with Precisely Controllable Mechanical and Degradation Properties
Researchers have recently developed a novel hydrogel material with precisely tunable mechanical properties and degradation rates through covalent crosslinking of hyaluronic acid with polyethylene glycol diamine. This technology substantially enhances the mechanical strength and stability of hyaluronic acid, opening new avenues for its biomedical applications.
Polyethylene glycol, with its excellent biocompatibility and hydrophilicity, serves as an ideal B. перекрестная увязкаagent. Its flexible molecular chains impart elasticity to the gel, while hyaluronic acid provides structural support, synergistically forming a high-performance gel system.
The study indicates that adjusting the crosslinker's molecular weight and crosslinking density effectively modulates the gel's elastic modulus and degradation behaviour. At 20% crosslinking density with a lower molecular weight crosslinker, the material exhibits its highest elastic modulus and slowest degradation rate, demonstrating outstanding comprehensive performance.
This customisable property renders the material particularly suitable for advanced medical applications such as drug delivery, cell transplantation, and tissue engineering, promising to advance the development and application of next-generation biomaterials.
2.2 Novel Hydrazide-Crosslinked Hyaluronic Acid Gels Enable Controlled Drug Release and Enhanced Enzyme Resistance
Researchers have recently developed a novel hydrogel exhibiting superior enzyme resistance and controlled drug release capabilities through efficient crosslinking of hyaluronic acid with polyhydrazide compounds. This technology significantly enhances the material's mechanical properties and stability, offering an innovative solution for controlled drug delivery systems.
Research indicates that by selecting different hydrazide crosslinking agents and adjusting reaction conditions, the physicochemical properties of the gel can be flexibly regulated. The resulting crosslinked gel exhibits good resistance to hyaluronidase, with degradation occurring only at the material interface and independent of crosslinker concentration, demonstrating a unique surface erosion degradation mechanism.
The gel maintains structural stability in acidic environments but undergoes gradual dissolution under alkaline conditions at pH values exceeding 7.0. This pH-responsive characteristic renders it particularly suitable for controlled drug release in alkaline environments. Furthermore, stable HA-ADH derivatives can be synthesised in the presence of high concentrations of cross-linking agents such as dihydrazine dihydrate, significantly enhancing the gel's mechanical rigidity while moderately reducing its water solubility. This enables sustained drug release while maintaining excellent biocompatibility.
This breakthrough offers novel material options for targeted drug delivery, tissue engineering, and intelligent biomaterial development, presenting broad clinical application prospects.
2.3 Novel Disulphide-Crosslinked Hyaluronic Acid Gel Facilitates Tissue Repair and Wound Healing
Recently, a research team successfully developed a novel hyaluronic acid gel (HA-DTPH) with sustained-release degradation properties and excellent biocompatibility using disulphide crosslinking technology. The degradation rate of this material can be flexibly adjusted by varying the crosslinking density, demonstrating broad application prospects in surface wound healing and tissue repair.
The gel was synthesised by reacting hyaluronic acid with 3,3′-dithiopropionic acid (DTP) under specific pH conditions, catalysed by ethylenediamine (EDC), yielding a structurally stable thiolated hyaluronic acid derivative (HA-DTPH). Research indicates that the disulphide-based B. перекрестные связиnetwork enables the gel to exhibit slow, controllable degradation behaviour both in vivo and in vitro, effectively prolonging the material's retention time at the site of action.
Owing to its tunable degradation properties and excellent biocompatibility, HA-DTPH gel holds promise as an ideal choice for next-generation wound dressings and tissue engineering scaffolds, offering innovative solutions for clinical skin repair, regenerative medicine, and related fields.
2.4 Innovative Esterification Technology Expands Hyaluronic Acid Applications, Empowering Diverse Biomaterials Development
By precisely controlling the esterification reaction between carboxyl and hydroxyl groups, hyaluronic acid derivatives with customisable esterification degrees can be synthesised. As the esterification degree increases, product water solubility progressively decreases while forming stable colloidal network structures that enhance resistance to enzymatic degradation. This product series exhibits excellent adaptability for film formation, fibre production, and microsphere preparation. Conventional processes such as freeze-drying and spray-drying enable fabrication into diverse forms, including sponges, fibres, films, and microspheres, suitable for applications such as sustained-release drug carriers, anti-adhesion coatings, and cell culture scaffolds.
Moreover, internal esterification technology further expands hyaluronic acid's application forms. The resulting lactone derivatives exhibit outstanding structural stability and biocompatibility, offering novel raw material options for tissue repair and regeneration materials.
2.5 Graft Modification Technology Drives Innovation in Hyaluronic Acid-Based Functional Materials
Hyaluronic acid can be combined with natural or synthetic polymers via graft modification techniques, forming novel functional materials with enhanced mechanical properties and physicochemical characteristics, thereby expanding its application potential across multiple fields.
Such materials typically involve chemically modifying hyaluronic acid using cross-linking agents (e.g., diazohydrazide) to generate derivatives (e.g., HA-ADH). The retained active groups within these molecules enable controlled grafting with functional molecules, constructing structurally stable, performance-tunable composite systems. This process facilitates intramolecular or intermolecular cross-linking while introducing diverse functional components, forming material platforms with customisable properties.
Graft-modified hyaluronic acid materials exhibit excellent biocompatibility and processability, finding applications in premium cosmetics, functional care products, carrier systems, and other industrial sectors demanding high biocompatibility, demonstrating broad application prospects.
2.6 Breakthroughs in Hyaluronic Acid Composite Modification Technologies Empower Development of High-Performance Biomaterials
Significant progress has recently been achieved in composite modification techniques based on hyaluronic acid. By combining hyaluronic acid with diverse natural or synthetic polymeric materials, researchers have successfully developed novel functional materials exhibiting superior mechanical properties, cell affinity, and controllable degradation characteristics, substantially broadening their application scope.
Hyaluronic acid composites with collagen effectively enhance material mechanical strength and structural stability. The CS-Gel-HA material formed by combining with the chitosan-gelatin system significantly boosts cell adhesion and proliferation activity, promoting normal cellular growth cycles. When combined with biodegradable polymers such as polylactic acid/polylactic-co-glycolic acid (PLA/PLGA), it maintains excellent biocompatibility while slowing the degradation rate of hyaluronic acid, prolonging its efficac,y and improving the material's processing and moulding capabilities.
Owing to their customisable physical, chemical, and biological properties, these composite materials demonstrate broad prospects in tissue engineering, premium skincare products, medical dressings, and regenerative medicine materials.
3. The application domains of modified hyaluronic acid materials continue to expand, becoming a focal point for innovation across multiple industries
With the continuous maturation of modification techniques, hyaluronic acid and its derivatives, leveraging their customisable superior characteristics, are increasingly being applied across numerous high-end biomaterial fields. Through targeted structural modifications and composites, hyaluronic acid materials can achieve enhancements in mechanical strength, adhesion, degradation cycles, and biocompatibility, meeting stringent requirements across diverse applications.
Currently, these materials have achieved breakthroughs in anti-adhesion, joint health, eye care, drug delivery, and tissue engineering, becoming crucial functional ingredients driving innovation and upgrades in related products. Learn More About Our Comprehensive Traceable, Consistent, Hyaluronic Acid Raw Material Solution.
4. Continuous Breakthroughs in Hyaluronic Acid Modified Materials Research with Broad Application Prospects
As a natural biomaterial, hyaluronic acid has become a vital ingredient in biopharmaceuticals and high-end medical devices due to its exceptional moisturising properties, biocompatibility, degradability, and unique rheological characteristics. Through various modification techniques such as cross-linking, esterification, grafting, and compositing, its mechanical properties, degradation resistance, and functional adaptability can be significantly enhanced, effectively expanding its application scenarios.
Green Spring Technology leverages advanced processes and stringent quality control to provide clients with a comprehensive range of high-quality hyaluronic acid raw materials and derivatives. Our products offer exceptional batch consistency and customisability, enabling clients to enhance product performance, shorten R&D cycles, and accelerate application deployment in advanced fields such as anti-adhesion, drug delivery, tissue engineering, ophthalmology, and orthopaedics.
Currently, modified hyaluronic acid materials demonstrate broad potential across multiple medical and health sectors. Looking ahead, as material properties and intelligent capabilities continue to advance, hyaluronic acid-based biomaterials are poised to deliver further innovative solutions for the industry. Green Spring Technology invites you to explore the boundless possibilities of hyaluronic acid together. Should you have relevant requirements or collaboration interests, we welcome your enquiries.
Contact us at helen@greenspringbio.com or WhatsApp: +86 13649243917 to learn more about technical details and application case studies.
Ссылка на сайт
[1] вайсманн B, мейер, В то же время structure of Гиалоби-уроническая acid and of hyaluronic acid from Пуповина [J]. - J. Новости компании Am По химии и химии Soc,1954,76(7):1753-1757.
[2] саари - эйч. Дифференциальный диагноз: Последствия для окружающей среды of Реакция на изменение Кислород в воздухе Сюл - - В чем дело? on Москва (Россия) Ii. Общая информация - жидкость and - очищенные, очищенные human Э-э... - с чернилами Спинной мозг, Воспаление,1993,17(4): 403-415.
[3] Джон О. механическая обработка properties and degradation Будь-гавьер of - гиалурони. acid Гидрогели (гидрогели) cross-linked На различных форумах cross-linking Плотность [J]. Углеводы (углеводы) Poly — РВК,2007,10:1-7.
[4] Пан хонгмей. Обзор текущего состояния исследований гиалуроновой кислоты [J]. Питание и ферментация сычуана, 2003, 39(1): 1-5.
[5] J E Scott, C Cummings, A Brass, et al. Вторичные и третичные структуры гиалуронана в водном растворе, исследованные вращающейся теневой электронной микроскопии и компьютерным моделированием [J]. Bochemial Journal, 1991, 274(3): 699-705.
[6] Evered D, wilan J. The biology of hyaluronan, ciba foundation symposium [J]. Джон уайли и Сыновья, 1989, 143: 6 — 15.
-
Предыдущий
Green Spring Technology's Hyaluronic Acid Powder Enhances Oral Beauty Product Formulations
-
Следующий проект
Hyaluronic Acid: A Multifunctional Natural Ingredient to Boost Your Product Innovation


 Английский язык
Английский язык Французский язык
Французский язык На испанском языке
На испанском языке Русский язык
Русский язык Корейская народно-демократическая республика
Корейская народно-демократическая республика На японском языке
На японском языке





