Как обездвижить папин порошок?
- папа! [EC 3.4.22.2] is an important biochemical product with widespread applications in the food and pharmaceutical industries, as well as in feed, textiles, and leather processing [1]. Due to the high cost of papain and its inability to be reused, researchers have been exploring the preparation of immobilized papain. This research began in the early 1960s and has since been extensively documented in the literature [2]. The main methods for enzyme immobilization include embedding, adsorption, covalent bonding, and cross-linking, each with its own advantages and disadvantages [3]. This paper summarizes recent research and application progress on immobilized papain, compares the enzymatic characteristics such as enzyme activity, stability, and recovery rate of immobilized papain prepared using various immobilization methods in recent years, and provides suggestions.
1 метод встраивания
Embedding method: The method of embedding enzymes or enzyme-containing bacterial cells in a porous carrier to fixate enzymes is called the embedding method. Gel embedding is a method of embedding enzymes in the microporous structure of various gels to produce immobilized enzymes of a specific shape. The most commonly used gels include agar, agarose, calcium alginate, carrageenan, and polyacrylamide. Microcapsule embedding involves encapsulating the enzyme within a semipermeable polymer membrane to produce microcapsule-immobilized enzymes.
1.1 альгинат-читосан иммобилизованный папайн натрия (ипсак)
Альгиническая кислота извлекается из коричневых водорослей и представляет собой полисахарид, состоящий из глюкуроновой кислоты, связанной цепями β 1,4. Это идеальный носитель для инкапсуляции фермента, с простым методом иммобилизации, мягкими условиями и операциями, которые могут быть проведены при комнатной температуре, что приводит к минимальной активации фермента. Однако гели альгината кальция нестабильны в растворах, содержащих поливалентные анионы (фосфаты, цифраты, лакты и т.д.) и высокие концентрации электролитов (K+, Na+), при этом ионы Ca2+ легко отделяются, что приводит к ослаблению или даже растворению геля. Он и др. [4] изучали иммобилизацию папайна с использованием альгината-читосана натрия в двух аспектах: во-первых, они использовали буфер трис-соляной кислоты во время подготовки геля для уменьшения отслойки ка и поддержания стабильности геля; Во-вторых, они впервые добавил читосан порошок для повышения твердости геля. Положительные заряда (-NH₂) на читосан взаимодействуют с карбоксильными группами альгината, стабилизируя гель, что делает его пригодным для загрузки колонн. Ферментная активность иммобилизованного фермента была такой же, как и фермента без порошка читина.
В He et al.'s [4] исследование, оптимальное значение pH для па и IPSAC было 7,2, и активность оставалась стабильной при температурах ниже 70 градусов. Пространственная структура встроенной па не претерпела изменений, поэтому оптимальное значение pH и теплостойкость остались практически неизменными. Период полураспада ипсак составляет 59 дней.
Альгинат-читосан имфрицированный папайн (IPSAC) обладает хорошей термостойкостью, длительным периодом полураспада и высокой твердостью геля, что делает его пригодным для загрузки колонн и удобным для автоматизированного производства в промышленных условиях.
1.2 метод капсулы читосан для иммобилизации папайна
Chitosan is a biopolymer obtained by deacetylation of chitin, also known as deacetylated chitin. As an enzyme carrier, chitosan has advantages such as chemical stability and good thermal stability, making it an excellent carrier for immobilized enzymes.
Хуанг зеюань [5] использовал читосан и карбоксиметил-целлюлозу натрия для формирования капсул иммобилизации папина, определяя оптимальные условия иммобилизации фермента следующим образом: загрузка фермента 500 мг /10 мл, концентрация читосана 0,2%, pH 7,5 и температура 50 градусов. Уровень восстановления активности иммобилированного папайна достиг 69,8%.
2. Метод адсорбции
Метод адсорбции: метод иммобилизации ферментов или ферментосодержащих бактериальных клеток путем адсорбции их на поверхность твердого адсорбента называется методом адсорбции. Общие адсорбенты включают активированный уголь, оксид алюминия, диатомную землю, пористую керамику, пористое стекло, силикагель и карбоксифосфат. Метод адсорбции для подготовки иммобилизованных ферментов прочен в эксплуатации, имеет мягкие условия, не вызывает денатурации или неактивации фермента, использует недорогие и легкодоступные переносчики и может быть использован повторно. Однако из-за слабых сил физической адсорбции ферментно-несущий комплекс нестабилен и подвержен отслоению, что ограничивает его практическое применение в промышленном производстве. Исследований в этой области также относительно мало.
2.1 метод адсорбции методом фриза-вакуума для иммобилизации папайна в мезопорных материалах
There are various methods for assembling functional materials in the pores of mesoporous molecular sieves, including gas-phase adsorption and impregnation. Compared to conventional adsorption methods, the freeze-vacuum adsorption method is a new approach with the following advantages: first, it provides a strong driving force, resulting in higher protein adsorption; second, it maintains enzyme activity at low temperatures.
Гао бо и др. [6] использовали мезопористый материал SBA-15 в качестве носителя для подготовки иммобилизованного папайна. Сравнивая характеристики иммобилизованных ферментов и ферментов раствора, они обнаружили, что оптимальная температура для папайна увеличилась с 60 до 70 градусов после иммобилизации. Оптимальное значение pH для фермента после иммобилизации составило 7,0, без изменения оптимального значения pH, которое может быть отнесено на счет нейтральных свойств поверхностного заряда мезопорных молекулярных ситов SBA-15. Некоторое количество иммобилизованного фермента и раствора фермента было взято, инкубация на 30-90 градусов в течение 30 минут, быстрое охлаждение до комнатной температуры, а затем фермент активность была измерена на 37 градусов.

После того, как папин был собран в молекулярные сивры, его тепловая стабильность значительно повысилась. Даже после инкубации на 90 градусов в течение 30 минут, он сохранил 70% своей активности, в то время как раствор фермент уже потерял свою активность на 90 градусов. Это говорит о Том, что сборка фермента в молекулярные сивры может повысить термоустойчивость. Поскольку высокие температуры не только повышают скорость реакции, уменьшают бактериальное загрязнение, но и повышают растворимость субструтов, повышая тем самым урожайность, иммобилизуемые ферменты, приготовленные с использованием мезопорового материала SBA-15 в качестве носителя, отличаются отличной термоустойчивостью, что делает их более пригодными для промышленного применения.
Экспериментальные результаты свидетельствуют о Том, что в соответствующих вакуумных условиях для подготовки иммобилизованных ферментов с высокой эффективностью сборки может использоваться метод адсорбции на основе фриза-вакуума, а активность фермента остается высокой с высокой стабильностью. Таким образом, адсорбция на основе холодного вакуума может служить новым методом иммобилизации ферментов белков.
3 метод ковалентного соединения
Метод ковалентной связывания: метод подготовки иммобилизованных ферментов путем ковалентной связывания несущественных групп за пределами активного центра фермента с группами на твердофазных несущих элементах называется методом ковалентной связывания, также известным как метод ковалентной связывания. Метод ковалентной связывания является одним из наиболее активно исследуемых методов иммобилизации в настоящее время. Его преимущества включают в себя крепкую связь между ферментом и носителем, сопротивление отслоению и способность непрерывно использоваться в течение длительных периодов времени.
Тем не менее, он также имеет следующие недостатки: (1) в процессе иммобилизации, прямые (жесткие) столкновения происходят между молекулами фермента и молекулами-носителями, которые могут изменить фермент 's конформация и причина отключения; (2) носителями, как правило, являются гидрофобные вещества. Когда ферменты непосредственно иммобилизованы на гидрофобных носителях, фермент ' микросреда s изменяется, что приводит к конформационным изменениям, складыванию или денатурации фермента белка; - неактивация; (3) традиционный метод ковалентного соединения приводит к иммобилизации ферментов с низкой свободой передвижения, и существует значительное стерильное препятствие между молекулами фермента и субструатами, что неблагоприятно для поддержания однородной каталитической активности свободного фермента. Поэтому этот метод называется "жесткая иммобилизация".
Распространенными носителями ковалентного соединения являются целлюлоза, декстран, agose и читин. Здесь мы сосредоточимся на нескольких новых методах ковалентной связи для иммобилизации папайна.
3.1 гибкая иммобилизация папайна с использованием крахмала бисальдозы
Для решения этих проблем с помощью методов ковалентной связи исследователи предложили прикреплять короткие цепи (молекулы мышц) к поверхности носителя для достижения "иммобилизации ферментов на основе руки". Однако, поскольку эти плечевые цепи являются короткими цепями с малым молекулярным весом и некоторые из них являются гидрофобными углеродными цепями, молекулы ферментов все еще испытывают значительные силы воздействия во время иммобилизации, что приводит к конформирующим изменениям и неактивации. Ван хайпин (Wang Haiping), Wei Rongqing (Wei Rongqing) и др. [7-8] предложили модель "гибкого иммобилизированного фермента", которая предусматривает прикрепление гидрофилических молекулярных цепей достаточной длины к иммобилизационному переносчику, именуемому "гибкими цепями", а затем привязку фермента к гибким цепям. Для обеспечения гибкой иммобилизации папайна, как показано на рис. 1, использовались следующие шаги.
На основе гибкой модели иммобилизации фермента был подготовлен гибкий носитель (chitosan-das50) с использованием крахмала chitosan и bis-aldehyde, а папайн был гибко иммобилизован. Уровень восстановления активности иммобилизированного фермента достиг 72%, что в три раза выше, чем у китозана-глутаральдегида (читосан-га). Гибкий иммобилированный папин сохранил половину своей деятельности после 7-8 партий и продемонстрировал значительно более высокую стабильность хранения, чем свободный фермент.
Полученные результаты свидетельствуют о Том, что метод "гибкой иммобилизации" не только снижает степень неактивации фермента в процессе иммобилизации, но и обеспечивает сохранение иммобилизованным ферментом более высокой однородной каталитической активности по сравнению со свободным ферментом.
3.2 иммобилизация папайна на магнитных полимерных микросферах
Магнитные полимерные микросферы представляют собой сверхтонкие порошки, содержащие магнитные металлы или оксиды металлов (такие как железо, кобальт, никель и их оксиды) и демонстрирующие магнитную чувствительность. Это новый функциональный полимерный материал, разработанный за последние два десятилетия. Различные реактивные функциональные группы (такие как гидроксильные, карбоксильные, альдегидные и аминогруппы) через химические реакции, такие как соколимеризация и модификация поверхности. Эти функциональные группы могут образовывать ковалентные связи с биоактивными веществами, такими как ферменты и антитела. Под влиянием внешнего магнитного поля эти микросферы могут подвергаться быстрому движению или разъединению. Поэтому магнитные полимерные микросферы, как новый вид функционального полимерного материала, имеют широкие перспективы применения в таких областях, как биомедицина, биоинженерия и иммобилизация фермента.
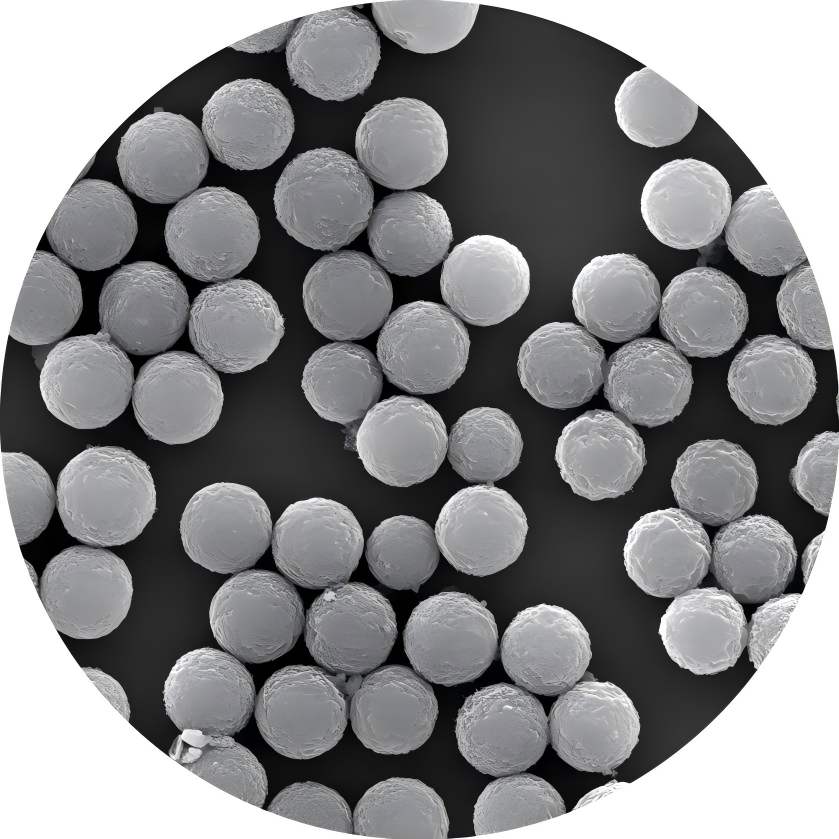
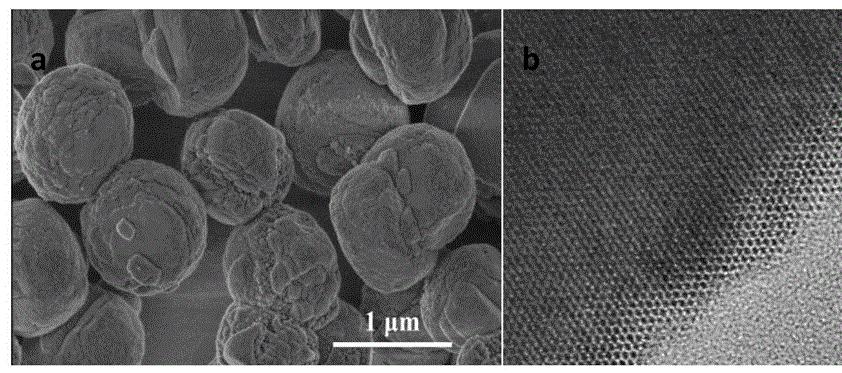
Zeng Lixi et al. [9] synthesized a highly hydrophilic, uniformly sized, well-dispersed magnetic polymer porous composite microsphere with a surface rich in functional epoxy groups, which was used for the immobilization of papain. Using casein as the substrate, the enzymatic properties and kinetic parameters of immobilized enzymes were studied. The results showed that the optimal reaction temperature for immobilized enzymes was 80°C. After incubation at 90°C for 40 minutes, the immobilized enzymes retained 65% of their original enzyme activity, while the solution enzymes only retained 6.6% of their original enzyme activity. The optimal reaction pH was 7.5. When the pH ranged from 6.0 to 8.5, the immobilized enzyme consistently exhibited higher activity than the free enzyme, with an enzyme activity recovery rate of 55.2%. The immobilized enzyme demonstrated better acid-base tolerance and significantly improved thermal stability, pH stability, and operational stability compared to the free enzyme.
Магнитно-полимерные пористые композитные микросферы иммобилизованного папаин, этот носитель обладает отличной гидрофилистичностью, биосовместимостью, большой поверхностью и высоким содержанием функциональных эпоксидных групп, что позволяет готовить высокоактивные и высокопроизводительные иммобилизованные ферменты. Используя магнитную чувствительность перевозчика, фермент может быть легко отделен от реакционной системы под внешним магнитным полем, что делает это тенденцией в использовании катализаторов для биохимических реакций.
3.3 иммобилизация папайна под руководством кареты хелатации металлов
Metal chelation affinity chromatography (IMAC) utilizes the coordination bonding between metal chelating ligands and electron-donating amino acids on the protein surface, such as the imidazole group of histidine and the thiol group of cysteine (with histidine being the most important), to achieve the separation and purification of proteins. Metal chelation carriers have minimal impact on the conformation of proteins immobilized on their surfaces and can be reused. Applying this technique to immobilized enzymes holds promise for overcoming some of the limitations of currently used immobilization carriers and methods.
Лиу линлин и др. [10] использовали магнитно-металлические хелирующие агрирующие микросферы в качестве носителей и использовали взаимодействие между металлическими хелирующими лигандами (IDA-Cu²⁺) и электронодонорскими аминокислотами на протеиновой поверхности для прямой иммобилизации папаина. Оптимальными условиями иммобилизации были Cu²⁺ 1.5 × 10^(−2) mol/g carrier, время иммобилизации 4 ч, иммобилизация pH 7.0 и фермент загрузки 30 мг/г carrier. Оптимальная температура реакции для иммобилизованного фермента составляет 70 градусов, а оптимальная реакция pH - 8,0. Тепловая стабильность иммобилизованного фермента значительно выше, чем фермента раствора. Восстановление ферментной активности иммобилизованного фермента составляет 68,4%, что свидетельствует о хорошей операционной стабильности. После пяти повторных видов использования носителя ферментационная активность иммобилизованного фермента составляет 79,71% от активности первоначально иммобилизованного фермента.

Результаты показывают, что при использовании металлических хелирующих носителей для целенаправленной иммобилизации папайна, состояние иммобилизационной реакции мягкое, с минимальным воздействием на фермент#39. Строение более высокого порядка и высокий уровень восстановления ферментной активности; Значительно улучшена тепловая стабильность иммобилизованного фермента; Перевозчик имеет высокую грузоподъемность фермента; Восстановление носителя и иммобилизация фермента просты в эксплуатации; Иммобилизованный фермент и носитель могут многократно использоваться повторно, а себестоимость иммобилизованного фермента является низкой. Поэтому данная технология имеет широкие перспективы применения в промышленности.
3.4 растворимый иммобилизованный папин
Иммобилизованные ферменты, приготовленные традиционными методами, имеют такие преимущества, как хорошая стабильность и удобная рекуперация, но их применение ограничено при работе с нерастворимыми высоковязкими субstrate растворами, что приводит к низкой каталитической эффективности. Низкая каталитическая эффективность ферментов объясняется ферментно-катализируемой реакцией, возникающей в неоднородной системе реакции между твердой и жидкой фазами, что приводит к значительному снижению вероятности контакта фермента и субструрата по сравнению с однородными системами свободной ферментной реакции. Поэтому основное внимание при повышении каталитической эффективности иммобилизованных ферментов следует уделять оптимизации системы каталитической реакции и подготовке иммобилизованных ферментов, способных сформировать однородную систему каталитической реакции.
Li Lingling et al. [11] conjuated papain (PP) с карбоксиметиловой целлюлозой ацетат сукцинат (asl) для подготовки растворимой в воде иммобилизованной PP. Оптимальные значения pH для свободной и растворимой в воде иммобилизованной PP были определены на уровне 6,0 и 5,0, соответственно, с оптимальной температурой реакции 60°C и 70°C, и значения км 2,53 и 3,07 мг · мл, соответственно. Растворимая иммобилизованная пп сохранила 62% своей деятельности после 12 часов инкубации на 60 ступенях, 62% своей деятельности сохранилось; При 4 градусах, через 30 дней, активность сохраняется на уровне более 90%; При pH 6,0 стабильность была наилучшей и оставалась относительно стабильной в диапазоне pH 4,0-7,0 (относительная активность > 80%). Растворимые иммобилизованные рр могут полностью растворяться в растворах с pH более 5,5, с более узким оптимальным диапазоном pH, более высокой оптимальной температурой и более высоким значением км; Значительно повышена тепловая и кислотная стабильность основания.
Растворимый иммобилизованный папин сочетает высокую каталитическую активность свободного папаина с высокой стабильностью и легкостью восстановления нерастворимых иммобилизованных ферментов, предлагая широкие перспективы применения в промышленности.
4 метод перекрестной увязки
Метод перекрестной увязки: метод перекрестной увязки предполагает использование бифункциональных реагентов для стимулирования перекрестной увязки между ферзимовыми молекулами или между ферзимовыми молекулами и твердофазными переносчиками для производства иммобилизованных ферментов. Широко используемые бифункциональные реагенты включают глутаральдегид, гексаметилентетрамин, малеиновый ангидрид и бис (2- нитро5 - нитрофенил) амин. Среди них наиболее широко используется глутаральдегид. Ферменты, иммобилизованные методом перекрестной связи, имеют высокую прочность связывания и могут использоваться в течение длительных периодов времени. Однако в силу активного характера перекрестной реакции многочисленные группы молекул фермента взаимосвязаны, что приводит к значительной потере ферментной активности. На практике этот метод часто сочетается с другими методами иммобилизации, такими, как инкапсуляция геля с последующим перекрестным соединением. Этот метод, в котором используются два или более методов иммобилизации, называется двойным или множественным иммобилизацией. С помощью этого метода могут быть подготовлены иммобилизованные ферменты с высокой ферментной активностью и хорошей механической прочностью.
Перекрестная увязка является наиболее часто используемым методом иммобилизации папайна. Исследования в этой области начались в китае в начале [12-13], поэтому мы перечислим лишь некоторые из последних исследовательских применений перекрестных методов иммобилизации папайна на новые носители.
4.1 безшелковый папайн
Чэнь фаньян и др. [14] использовали активированный шелковый фиброин в качестве перевозчика и использовали ковалентный метод перекрестной связи для иммобилизации папайна, изучая ферментативные свойства шелкового фиброина-иммобилизованного папайна. Результаты показали, что из-за того, что шелковый фиброин является гидрофилистическим полимером, иммобилизованный папен, приготовленный с использованием шелкового фиброина в качестве носителя, продемонстрировал значительно более высокую степень родства с субстритом. Видимое значение постоянной км приложения Michaelis [casein] иммобилизованного фермента составило 0,092%, что в 0,46 раза превышает значение фермента раствора (рис. 2); Оптимальный показатель pH составил 7,5; При значениях pH от 6,5 до 8,0 фермент остается стабильным в температурном диапазоне 4,0-55°C; Период полураспада раствора составляет 38 суток, а иммобилизованного фермента — 54 суток. Период полураспада иммобилизованного фермента значительно превышает период полураспада фермента раствора.
4.2 обездвиженный папин с использованием овальбумина в качестве носителя
Хуан йибинг и др. [15] использовали денатурированный овальбумин непосредственно в качестве носителя в сочетании с традиционными методами перекрестной иммобилизации и успешно иммобилизовали папайн, используя глутаральдегид в качестве связующего агента, упрощая оперативные этапы. Исследования показали, что иммобилизованный папайн с использованием овальбумина в качестве носителя проявляет оптимальные температуры реакции 60 градусов для местного фермента и 90 градусов для иммобилизованного фермента в тех же условиях реакции.
Увеличена оптимальная температура реакции иммобилизованного фермента. Оптимальное значение pH иммобилизованного папайна также изменилось в сторону щелочности до 8,0, а кислотная устойчивость папайна, иммобилизованного с использованием порошкового овальбумина в качестве носителя, значительно повысилась. После обработки в кипящей водяной ванне на 100 градусов в течение 5 часов, иммобилизованный папайн сохранил 54,6% своей деятельности, в то время как фермент раствора полностью потерял свою ферментатическую активность после 2,5 часов обработки. Экспериментальные результаты показывают, что иммобилизованный папайн демонстрирует повышенную стабильность в экологических условиях. Стабильность является важнейшим фактором, определяющим практическую применимость иммобилизованных ферментов. В большинстве случаев устойчивость фермента повышается после иммобилизации, что очень выгодно.
4.3 читосан-иммобилированный папайн
Читосан-иммобилированный папаин is currently a relatively mature method for immobilizing papain [16–18]. The main method involves using chitosan as a carrier and glutaraldehyde as a cross-linking agent to prepare immobilized papain [18]. Using chitosan as a carrier, the method for preparing immobilized papain is simple, with mild immobilization conditions, and the resulting immobilized enzyme exhibits significantly improved heat resistance and thermal stability, making it a commonly used method for immobilizing papain.
5 резюме и перспективы
Есть много методов для подготовки иммобилизованных папайн. Из этих обширных исследований можно сделать вывод о Том, что после иммобилизации ферменты претерпевают определенные изменения в своих характеристиках под воздействием носителей и других факторов. Поэтому при применении иммобилизованных ферментов важно понимать различия в свойствах между иммобилизованными ферзимами и свободными ферзимами, поскольку эти знания имеют решающее значение для оптимизации их применения. Благодаря соответствующей корректировке процесса ферменты могут функционировать в оптимальных условиях для эффективного катализации реакций. Из-за различий в экспериментальных конструкциях между исследователями трудно оценить преимущества и недостатки различных методов иммобилизации. Иммобилизованный папайн, подготовленный с использованием различных методов, имеет различные диапазоны применения, и соответствующий метод следует выбирать исходя из фактических потребностей для более эффективного обслуживания производства.
После иммобилизации с помощью различных методов, papain демонстрирует повышенную устойчивость, повышенную оптимальную температуру, более широкий диапазон pH терпимости, улучшенную ферментную активность и длительный период полураспада, что указывает на то, что метод иммобилизации является подходящим. Для достижения лучших результатов следует продолжать оптимизацию. Исходя из имеющихся результатов исследований, дальнейшее улучшение активности и субстратной аффилированности иммобилизованных ферментов является ключевым направлением будущих исследований.
Подводя итоги недавнего прогресса в исследовании иммобилизованного папайна, можно увидеть, что при постоянном развитии технологии каталитическая активность и стабильность иммобилизованного папайна являются удовлетворительными. Технология иммобилизации ферментов позволяет многократно использовать ферменты и эффективно разделять сырье, продукты и ферменты, тем самым упрощая процессы разделения и оборудование, сокращая производственные циклы и снижая производственные издержки. Иммобилизованные ферменты станут важной формой применения ферментов в промышленности. Повышение степени зрелости технологии иммобилизации фермента позволит папайну играть еще большую роль в производстве продуктов питания, напитков, фармацевтических препаратов, химических реагентов, кормов, текстильных изделий и косметики.
Ссылки на статьи
[1] джоне дж.г. утонченный папайн [дж]. Биохимия процессов, 1974, 9(6): 21-24.
[2] сюн хуа. Прогресс в области исследований по применению папайна [J]. Сохранение и переработка, 2006(1): 7-8.
[3] Yao X L, Ku H S, Song W J. исследование ферментативных характеристик иммобилизации трипсина с различными носителями [J]. Биотехнология, 2007, 17(3): 71-73.
[4] He Ping, Huang Zhuolie, Li Chuny, et al. Иммобилизация и свойства папайна [J]. Журнал тропической и субтропической ботаники, 2008, 16(4): 334-338.
[5] хуан зеюань. Исследование по иммобилизации папайна с использованием капсул читосан [J]. Наука и техника о продовольствии, 2002 год (12): 10-12.
[6] гао бо, жу гуаншан, фу сюэки и др. Иммобилизация папайна в мезопорных материалах с использованием адсорбции на основе холодного вакуума [J]. Журнал джилинского университета, 2005, 43(6): 66 — 71.
[7] ван хайпин, вей ронцин, шэнь бин и др. Исследование по вопросу о гибкой иммобилизации папайна с использованием крахмала бисальдозы [J]. Биохимическая инженерия, 2004, 2(1): 25-29.
[8] вей ронцин, шэнь бин, лю сяонин и др. Гибкая иммобилизация папайна с использованием Chitosan Carrier [J]. Журнал технологического инжиниринга, 2005, 5(2): 183 — 187.
[9] цзэн лиси. Исследование активности и стабильности папайна, иммобилизованного на пористых микросферах новых магнитных полимеров [J]. Журнал естественных наук, хунанский обычный университет, 2007, 30(1): 101 — 106.
[10] лю линлин, цзэн лиси, лю тинг и др. Исследование по вопросу о прямом иммобилизации папайна на металлические хелаты [J]. Журнал биоинженерии, 2005, 20(5): 26-31.
[11] ли линлинг, чжан тао, ю ронг и др. Подготовка и свойства растворимого иммобилизованного папайна [J]. Журнал West China Pharmacy, 2007, 22(2): 77-79.
[12] тао голян, ли янфэн. Исследование по иммобилизации папайна на обработанный аммонием макропористый сферический поливинилхлорид [J]. Прикладная химия, 1993, 10(2): 9-12.
[13] тан хюйин, чэнь сюэлин. Иммобилизация и инфракрасная активация папайна на микробусах [J]. Биотехнология, 1993, 3(5): 17-20.
[14] чэнь фаньян, чжи пинсян. Исследование характеристик шелкопрядного папайна [J]. Журнал южно-китайского сельскохозяйственного университета, 2005, 26(4): 81-83.
[15] хуан йибинь, у сяося, у шеньнань и др. Исследование по вопросу о иммобилизации папайна с использованием овальбумина в качестве носителя [J]. Журнал джилинского университета, 2004, 42(4): 15-20.
[16] хуан цзяньшао, чжан хон. Читосан-иммобилированный папайн [дж]. Журнал Changde Normal University, 2002, 14(1): 36-39.
[17] юань чутао, цзян сяньминь. Исследование по вопросу о читосан-г-акрилонитриле-иммобилизованном папайне [J]. Прикладная химия, 2002, 19(9): 28-31.
[18] ляо цю хуа. Исследование по иммобилизации папайна микрокристаллическим читосаном [J]. Журнал военно-медицинского колледжа гуанчжоу, 1998, 21(1): 30-32.
-
Предыдущий
Что такое Papain и его применение в кормовой промышленности?
-
Следующий проект
Какой смысл в обездвиженном папайне?


 Английский язык
Английский язык Французский язык
Французский язык На испанском языке
На испанском языке Русский язык
Русский язык Корейская народно-демократическая республика
Корейская народно-демократическая республика На японском языке
На японском языке





